Abstract
Introduction: Reduced intensity conditioning (RIC) allogeneic stem cell transplantation (ASCT) remains a potentially curative strategy in high-risk CLL. Post-ASCT relapse can be predicted by positive minimal residual disease (MRD) at 12m. We present the final results of a prospective trial on the efficacy and safety of a post-ASCT preemptive immunointervention (PrIm) based on serial MRD assessment in high-risk CLL (NCT01849939).
Material (or patients) and methods: Main inclusion criteria were (1) EBMT 2007 criteria for ASCT, (2) CLL in PR/CR, (3) mass ≤5cm, (4) age ≤70, (5) SORROR score ≤2 and (6) HLA donor (10/10). RIC regimen included fludarabine 120 mg/m2, IV busulfan 6.4 mg/kg, rabbit ATG 5mg/kg and CsA prophylaxy. Centralized 10-color flow cytometry blood MRD was assessed before and at M1, M2, M3, M4, M5, M6, M9 and M12 post-ASCT. MRD[-] was defined by blood detection <10e-4 at 2 consecutive time-points, confirmed on bone marrow aspiration. PrIm algorithm, applied from D30 to M12, considered IWCCL response criteria, blood MRD levels and GVHD occurrence/severity. PrIm comprised tutored CsA early (≤4-5m), standard (6m) or late (7m) tapering and discontinuation (T&D). Early CsA T&D was followed if failure by 1-3 increasing DLI doses (1, 5 or 10x10e6 CD3/kg). Primary end point was the rate of MRD[-] at M12.
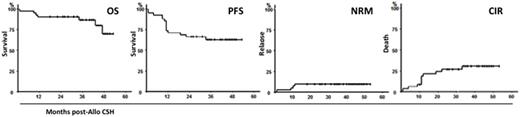
Results: The 43 planned inclusions were done between 09/2012 and 02/2015 in 16 centers. One patient had no ASCT for donor reason hence this analysis includes 42 pts, median age 58 y (range, 40-68). Before their last line prior to ASCT, patients had presence of del(17p) and/or TP53mut either in 1st line (26%) or in relapse (38%) or had absence of del(17p) and/or TP53mut but relapse ≤2y post fludarabine-based combination (36%). They had received a median of 2 (range 1-5) lines, including alemtuzumab in 21 and BCR inhibitor in 4. At time of transplantation, 21% had achieved CR and 79% a PR. Six patients had undetectable MRD. For the 36 other patients, the median pre-ASCT MRD level was 2.35% (0.014 to 70%).The MRD negativity MRD[-] was achieved at 12 m in 64% (27/42) and 39 pts could be grouped in 4 patterns according to MRD evolution: Pattern A (n=6) [pre-transplant MRD[-] remaining MRD[-] within 3m post-ASCT] ; pattern B (n=11) [Spontaneous conversion before from MRD[+] to MRD[-] within 3m] ; pattern C (n=15) [post-transplant MRD[+] who became negative after CsA T&D or after cGVHD] ; pattern D (n=7) [post-transplant MRD[+] remaining MRD[+] despite cGVHD or CsA T&D followed by DLI]. Deaths were recorded in 7 pts as the result of relapse with Richter syndrome in 3 pts and of toxicity in 4 pts : extensive cGVHD (n=2), pulmonary aspergillosis plus pneumocystose associated with limited cGVHD (n=1) and early CMV and EBV infection (n=1). Chimerism analysis revealed that absence of M1 full T-cell engraftment (<95% donor) was predictive of M12 MRD+ (p=0.036). Serial chimerism analysis revealed a poor M2 and M3 T-cell engraftment in Pattern D compared to pattern A, B and C (p=0,043 and p=0,006). The median follow-up of surviving pts is 36m [19-53]. Grade II and III aGVHD were seen in 6 and 3 pts. Cumulative incidence of limited extensive cGVHD at 2 y was 38% (95%CI 23%-53%) and 23% (95%CI 10%-36%) respectively. Two cGVHD were secondary to planned PrIm : one extensive after CSA T&D and one limited after DLI. Eleven patients had progression occurring at a median of 11.7m [1-34] post-ASCT translating into a 2-y progression of 33%. Relapse treatments, given at the discretion of physicians were ibrutinib in 8 pts, (including one with DLI before ibrutinib), DLI alone in 1 pt and RCHOP in 1 pt.Finally, the 2-y OS, PFS and NRM were 90.5% (95%CI 77.9%-96.2%), 66.7% (95%CI 51.5%-79%) and 7.1% (95%CI 3.1%-11.1%) respectively. (Figure).
Conclusion: These data highlight the feasibility, safety and efficacy of a standardized PrIm strategy in the context of allo-ASCT for high-risk CLL. The impact of early full T-cell engraftment on subsequent MRD negativity suggests the importance of an early intervention on MRD clearance.As DLI appears to have limited impact when CsA T&D fails, and based on recent published data focusing on ibrutinib post-ASCT, the preemptive use of such agents should be considered at this point for persistent MRD[+] after 3-6m post-ASCT.
Tournilhac: AMGEN: Other: Travel funding, Research Funding; GILEAD: Honoraria, Other: Travel Funding, Research Funding; Janssen: Honoraria, Other: travel funding; Abbvie: Honoraria, Other: Travel funding; ROCHE: Honoraria, Other: Travel funding, Research Funding. Guieze: GILEAD: Other: Educational Presentation; JANSSEN: Other: Educational Presentation; ABBVIE: Other: Educational Presentation. Leblond: Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria; Novartis: Honoraria; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.